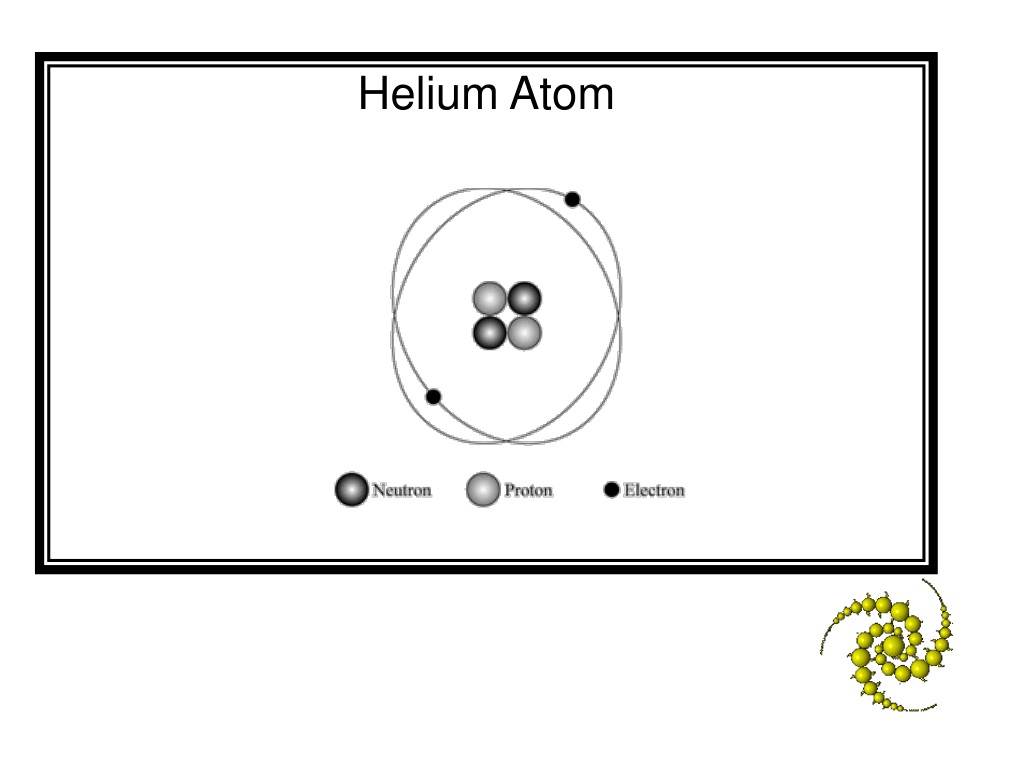
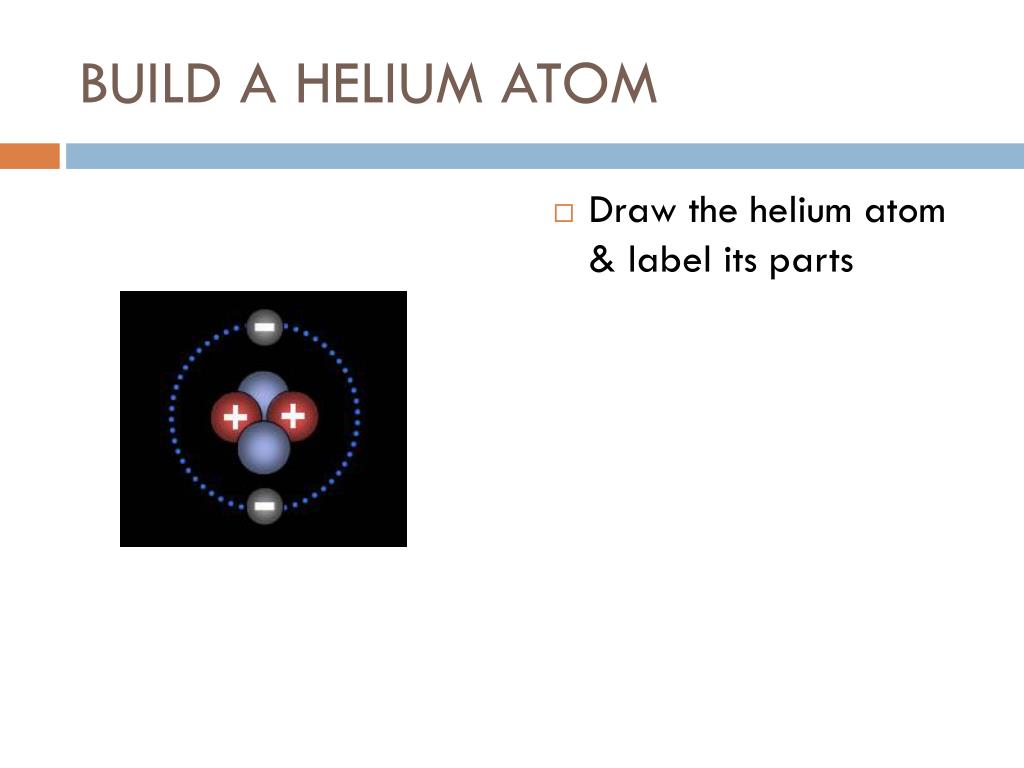
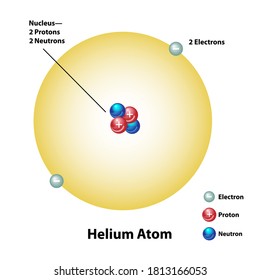
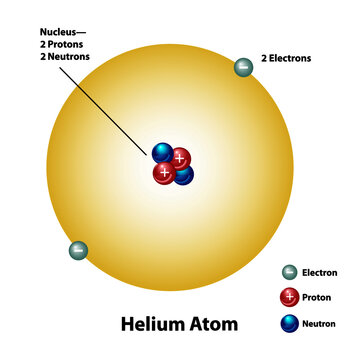
45 label the parts of the helium atom.
Helium atom - Wikipedia A helium atom is an atom of the chemical element helium.Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with either one or two neutrons, depending on the isotope, held together by the strong force.Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found. PDF Label The Parts Of A Helium Atom Pdf Free Draw A Circle To Represent The Nucleus Of The Atom. 2. Write The Element's Symbol, Number Of Protons (p) And Number Of Neutrons (n) Inside The Circle. 3. Draw Rings Around The Circle To Represent Electron Shells. Each Ring Represents A Dif Jul 15th, 2022 The Atom The Electrons In The Atom Homework From The …
The Periodic Table Questions and Answers | Homework.Study.com An atom of an element has a mass ever so slightly greater than twice the mass of a Ni atom. Identify the element. View Answer. Identify the group, family, or other periodic table location of the element with the given outer electron configuration. ns2np3 . View Answer. Account for the element and the part of the periodic table in which the element with the mentioned …
Label the parts of the helium atom.
Helium Atom Diagram Labeled - Bohr’s Atom | Julia Biermann Question 21 draw the general structure of a helium atom and label: Neutron, proton, shell/energy level, nucleus, electron. The nucleus contains uncharged neutrons and positively charged protons, whereas the orbiting electrons possess negative charges. Two protons are present in the nucleus of all helium atoms. helium | Definition, Properties, Uses, & Facts | Britannica helium (He), chemical element, inert gas of Group 18 ( noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F). The boiling and freezing points of helium are lower than those of any other known substance. Helium (He) - Physical & Chemical Properties, Uses, Isotopes - BYJUS Helium has two known stable isotopes - 3 He and 4 He. The abundance of helium-3 and helium-4 corresponds to 0.0002% and 99.9998% respectively. This difference in abundances can be observed in the Earth's atmosphere, where the ratio of 4 He atoms to 3 He atoms is approximately 1000000:1. Physical Properties of Helium Chemical Properties of Helium
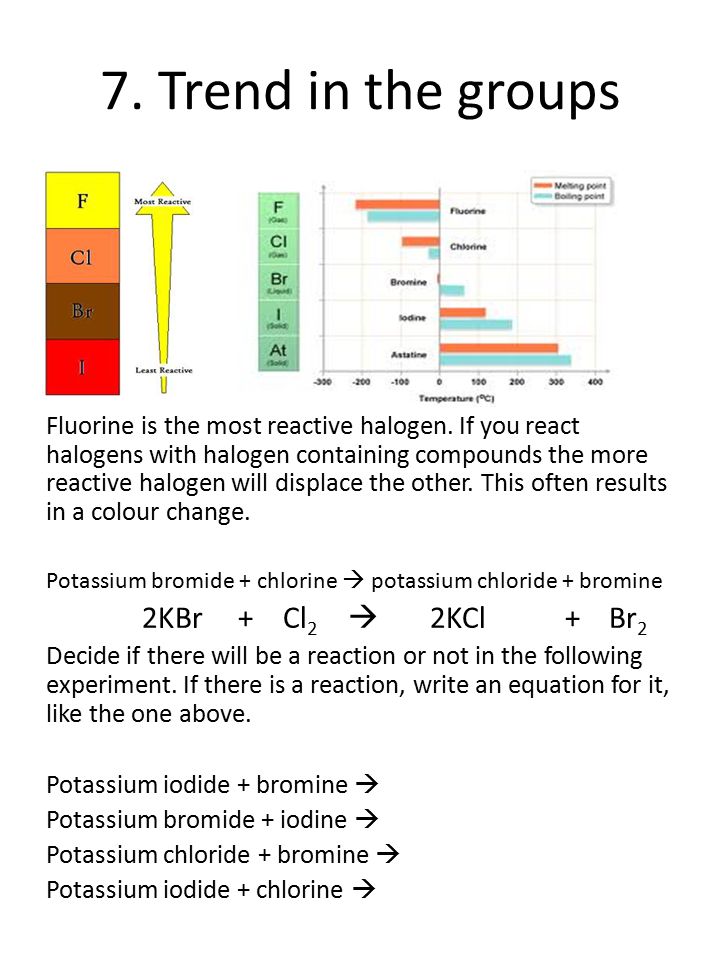
Label the parts of the helium atom.. PDF Helium Atom, Approximate Methods - University of Delaware The Helium Atom and Variational Principle: Approximation Methods for Complex Atomic Systems The hydrogen atom wavefunctions and energies, we have seen, are deter-mined as a combination of the various quantum "dynamical" analogues of classical motions (translation, vibration, rotation) and a central-force inter- Wave function for a helium atom | Physics Forums For helium, you should be doing the same, namely first rewrite the Hamiltonian in the center-of-mass frame, then look for solutions in terms of the relative coordinates. Note also that the label "proton" is not appropriate for helium, but should rather be "nucleus." Oct 10, 2019 #3 Gold Member 507 32 DrClaude said: How to draw Bohr diagram for Helium(He) atom - Topblogtenz The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be found by subtracting the number of protons from the atomic ... Pink Floyd - Wikipedia Pink Floyd are an English rock band formed in London in 1965. Gaining an early following as one of the first British psychedelic groups, they were distinguished by their extended compositions, sonic experimentation, philosophical lyrics and elaborate live shows.They became a leading band of the progressive rock genre, cited by some as the greatest progressive rock band of all time.
Atomic Structure - Rim of the World Unified School District How many electrons are in the nucleus of an atom with an atomic number of 20? How many neutrons are in the nucleus of an atom with an atomic number of 25? (use Periodic Table for mass) What is the mass number of an atom with 3 protons, 4 neutrons, and 3 electrons? How many neutrons are in the nucleus of an atom that has an atomic mass of 36 and an Loading... - BrainPop Loading... - BrainPop ... Loading... Lava Service Centres List, Customer Care, Spare Parts Price Lava International has an extensive network of service centres and pick-up points across India. In the rare case, you experience a problem with your purchase you can quickly locate a Lava service partner from the above menu. We have also facilitated a list of genuine spare parts to ease your concerns. PDF Helium 'Atom' - SparkFun Electronics The Atom is a dual band FCC, ETSI and IC certified IEEE 802.15.4 module. It operates in the 2.4GHz and 915MHz bands in North America and 2.4GHz and 868Mhz bands in Europe. The module is also capa-ble of supporting China frequencies. The module is designed to provide wireless con-nectivity to the Helium network infrastructure via
What Are The Parts Of An Atom? - Universe Today When an element undergoes decay, its nucleus loses energy by emitting radiation - which can consist of alpha particles (helium atoms), beta particles (positrons), gamma rays (high-frequency... Answered: 11.Label the parts of the helium entry… | bartleby A: mass of an average Chromium atom =51.9961 u Now we have to calculate 38.8% of the mass of an average… Knowledge Booster Learn more about Introduction to Organic Chemistry 5. Helium atom(s) (27 pts.) a. The electron | Chegg.com Be sure to label the spin and spatial functions with the electron numbers (1 or 2). (3 pts.) f. What are the allowed values of S and Ms for a single one of these Helium atoms in the triplet (1s)1(2s)2 electron configuration? (3 pts.) g. Assume the two triplet Helium atoms are brought into proximity and experience spin-spin coupling. Properties of Periodic Table of Element Groups - ThoughtCo 01.04.2016 · There are multiple ways of grouping the elements, but they are commonly divided into metals, semimetals (metalloids), and nonmetals. You'll find more specific groups, like transition metals, rare earths, alkali metals, alkaline earth, halogens, and noble gasses.
Atomic Design Methodology | Atomic Design by Brad Frost Each atom in the natural world has its own unique properties. A hydrogen atom contains one electron, while a helium atom contains two. These intrinsic chemical properties have profound effects on their application (for example, the Hindenburg explosion was so catastrophic because the airship was filled with extremely flammable hydrogen gas versus inert helium gas). In the …
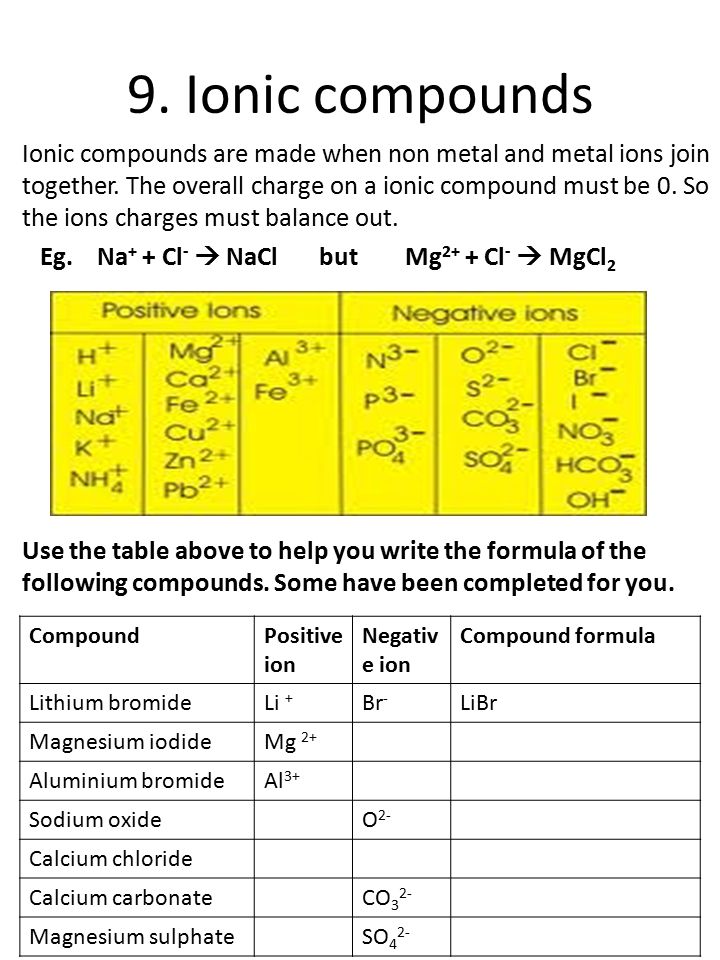
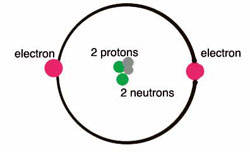
PDF C£ ot- COiVfo d Label parts A, B, and C on the helium atom model above 4. Write the word from Column B inthe space before its description in Column A. ColumnA Tht{'l\tCJc \\l)Sa. coined the term "atom" Q__\.Q_~ b. orbit inlayers.f\\.J G\-tU S c.most of the mass of an atom 120.1iz>Y\.. d. modern atomic theory
Helium - Periodic Table Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
1.8: Helium Atom - Chemistry LibreTexts The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as (1.8.1) He → I 1 He + + e − → I 2 He + + + 2 e − The first ionization energy I1, the minimum energy required to remove the first electron from helium, is experimentally 24.59 eV.
Basic Chemistry Tutorial 2, Drawing Atoms - learn-biology Basic Chemistry for Biology, Part 2: Drawing Atoms Using the Octet Rule. 1. Drawing the Simplest Atoms: Hydrogen and Helium. The most common element in the universe is hydrogen, a gas that makes up about 99% of the universe's known mass 1. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system.
Atomic Structure Quiz Flashcards | Quizlet Atomic Structure Quiz. Draw and Label the parts of a helium atom. Include the mass and charge of each subatomic particle. Should have two protons and two neutrons and 2 electrons and an electron cloud. protons: +, 1 amu neutrons: 0, 1 amu electrons: -1, 1/1840 amu.
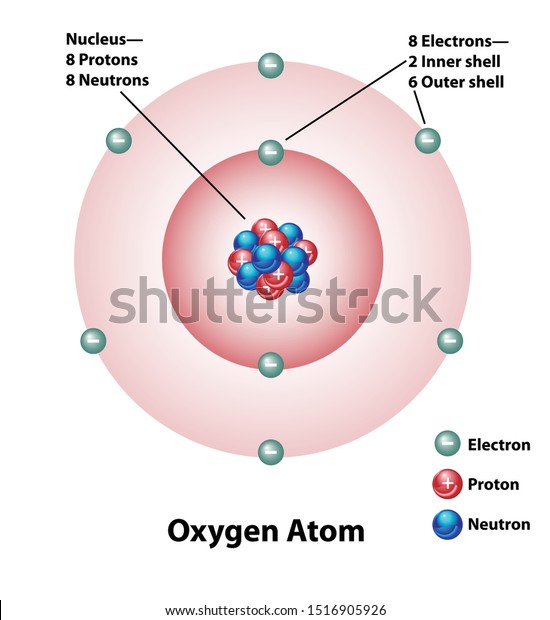
Elements and Compounds Printable Worksheets - TeAch … The basic building block of matter is an atom. Two or more atoms combine to form molecules. Elements and compounds are both made up of atoms. An element has only one kind of atom, whereas compounds contain different atoms. An oxygen molecule has two oxygen atoms. Whereas the compound, carbon dioxide, has two carbon atoms and one oxygen atom.
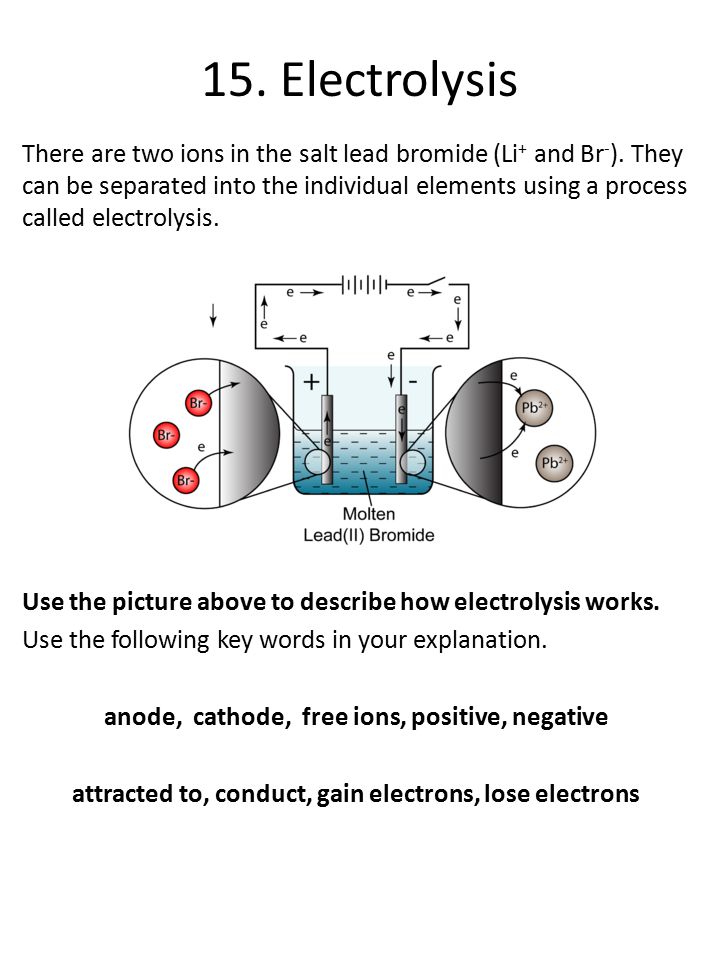
Helium - Wikipedia Helium (from Greek: ἥλιος, romanized: helios, lit. 'sun') is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling and melting point are the lowest among all the elements.It is the second lightest and second most abundant element in the ...
Helium - Element information, properties and uses | Periodic Table Some of the helium formed escapes into the atmosphere, which contains about 5 parts per million by volume. This is a dynamic balance, with the low-density helium continually escaping to outer space. It is uneconomical to extract helium from the air. The major source is natural gas, which can contain up to 7% helium.
draw and label the parts of a helium atom - wallpaperpchdcute How to Draw amp Label the Parts of a Helium Atom www. The circle represents the nucleus of a helium atom. Draw two small letter es on the outer circle to represent the helium atoms two electrons in orbit around the nucleus. The center of an atom is the nucleus and one or more electrons surrounding the. 1 amu neutrons.
PDF Mr. E. Science Label the parts of the helium atom pictured below, WORD BANK neutron electron 1991 Instructional Fair, Inc. proton orbit (shell) Earth Science IF8755 nucleus . Protons, Neutrons and Electrons Name The atomic number of an atom is the number of protons in each atom of that element. Because atoms are electrically neutral, the atomic number is also the
Periodic Table of Elements: Los Alamos National Laboratory Jules Cesar Janssen obtained the first evidence of helium. Diagram of a helium atom. There are only two electrons orbiting helium's nucleus. Helium ballons are lighter than air. ... The helium content of the atmosphere is about 1 part in 200,000. While it is present in various radioactive minerals as a decay product, the bulk of the Free World ...
PDF Mrs. Parsiola's Homepage - Home dubnium 2 (262) carbon 12.01 15 cobalt 58.9332 rhodium 102.905 iridium 192.2 109 meitnerium 2 (266) nickel 58.69 palladium 106.4 platinum 195.09 110 2 ununnilium i (269) chromium 51.996 molybdenum 95.94 tungsten 183.85 106 2 seaborgium 2 (263) manganese 54.9380 technetium (98) rhenium 186.2 107 bohrium (262) word bank element's symbol element's …
2649: Physics Cost-Saving Tips - explain xkcd 22.07.2022 · He. It therefore follows that decay from a helium-2 atom to two hydrogen-1 atoms would release 1.25 MeV, [citation needed] per the conservation laws of energy and mass. A moderately-sized balloon might have a diameter of 12 inches. Some calculations give this a volume of roughly 14.83 liters (assuming a spherical balloon.)
Helium - Protons - Neutrons - Electrons - Electron Configuration Helium-3 is a light, stable isotope of helium with two protons and one neutron (the most common isotope, helium-4, having two protons and two neutrons in contrast). Other than protium (ordinary hydrogen), helium-3 is the only stable isotope of any element with more protons than neutrons. Helium-3 was discovered in 1939.
8: The Helium Atom - Chemistry LibreTexts The helium atom has two electrons bound to a nucleus with charge Z = 2. The successive removal of the two electrons can be diagrammed as. (8.1) He → I 1 He + + e − → I 2 He + + + 2 e −. The first ionization energy I1, the minimum energy required to remove the first electron from helium, is experimentally 24.59 eV.
Hydrogen - Wikipedia Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2.It is colorless, odorless, tasteless, non-toxic, and highly combustible.Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal …
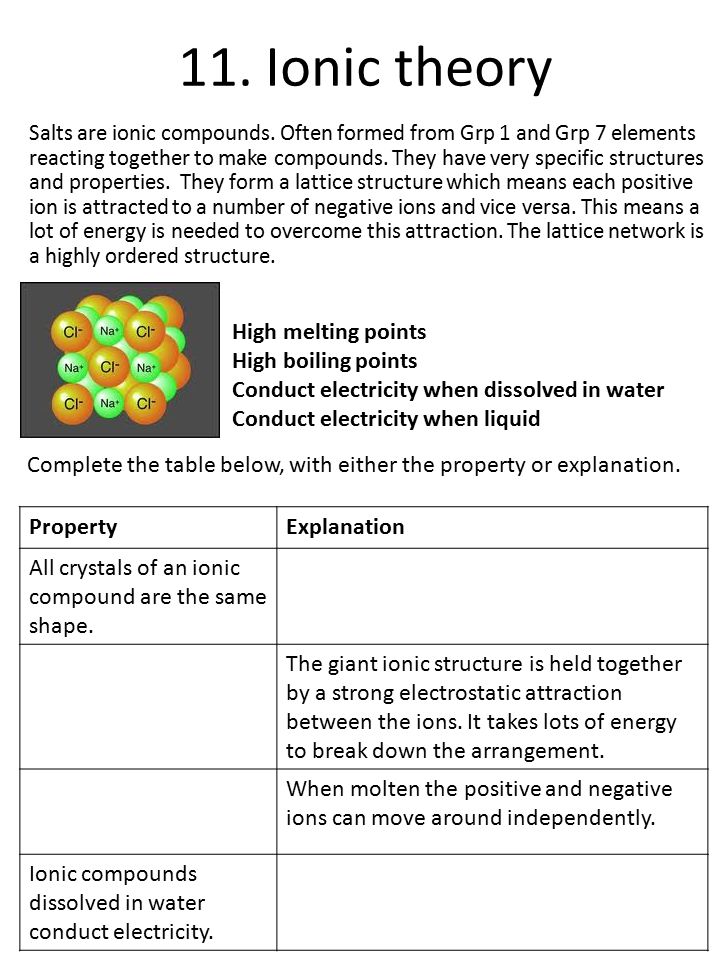
The Structure of an Atom Explained With a Labeled Diagram The atomic model in the diagram below shows protons and neutrons concentrated at the atomic nucleus and electrons in the orbits surrounding it. Protons are positively charged, electrons are negatively charged, while the neutrons carry no charge. J.J. Thomson Plum Pudding Model
Atomic Energy Level Diagrams - GSU Energy level diagrams can be useful for visualizing the complex level structure of multi-electron atoms. Forms of such diagrams are called Grotrian diagrams or term diagrams in various parts of the literature. While the energy level diagram of hydrogen with its single electron is straightforward, things become much more complicated with multi ...
Helium (He) - Physical & Chemical Properties, Uses, Isotopes - BYJUS Helium has two known stable isotopes - 3 He and 4 He. The abundance of helium-3 and helium-4 corresponds to 0.0002% and 99.9998% respectively. This difference in abundances can be observed in the Earth's atmosphere, where the ratio of 4 He atoms to 3 He atoms is approximately 1000000:1. Physical Properties of Helium Chemical Properties of Helium
helium | Definition, Properties, Uses, & Facts | Britannica helium (He), chemical element, inert gas of Group 18 ( noble gases) of the periodic table. The second lightest element (only hydrogen is lighter), helium is a colourless, odourless, and tasteless gas that becomes liquid at −268.9 °C (−452 °F). The boiling and freezing points of helium are lower than those of any other known substance.
Helium Atom Diagram Labeled - Bohr’s Atom | Julia Biermann Question 21 draw the general structure of a helium atom and label: Neutron, proton, shell/energy level, nucleus, electron. The nucleus contains uncharged neutrons and positively charged protons, whereas the orbiting electrons possess negative charges. Two protons are present in the nucleus of all helium atoms.


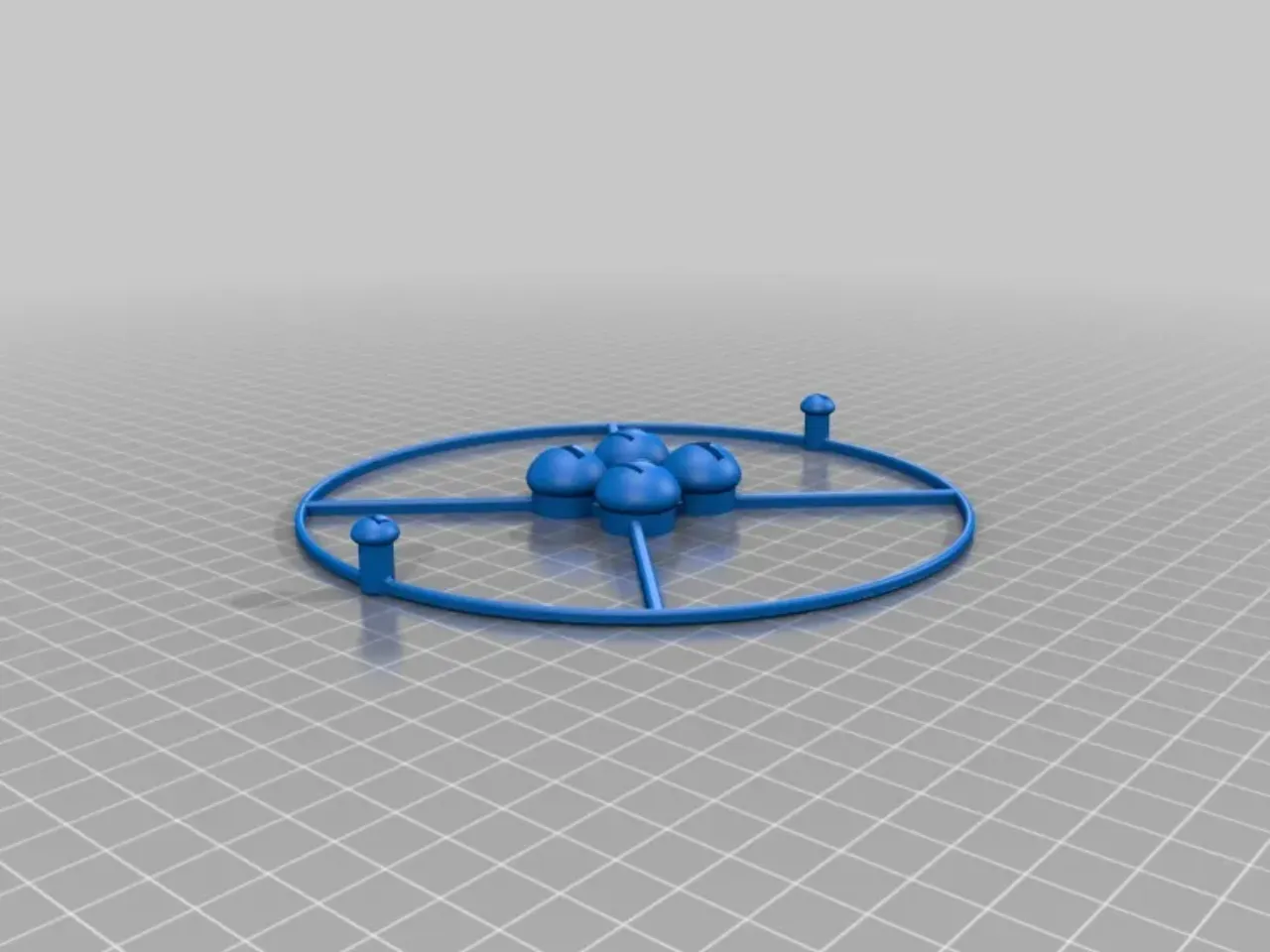






![Helium Atom - 3D model by Carlos (@carlossamuelariza) [134435c]](https://media.sketchfab.com/models/134435c52f804925a852d969c206eec3/thumbnails/e5246be4a42640e7b80c1f3e8ed6c687/f79bee2380454aeb932ee6e94ff0e385.jpeg)


















Post a Comment for "45 label the parts of the helium atom."